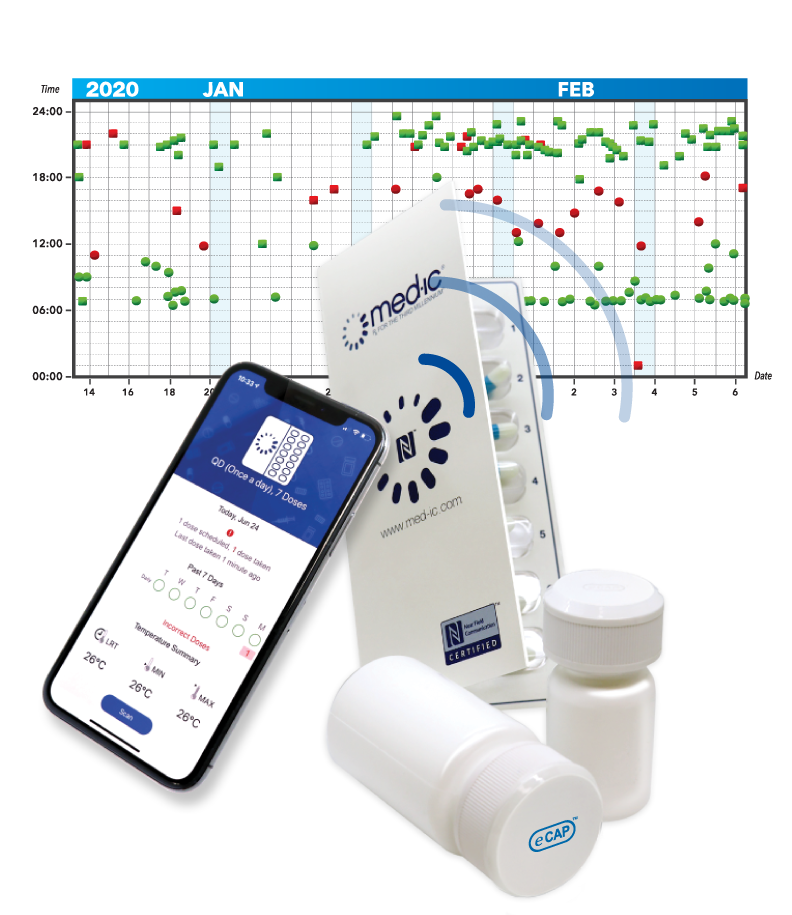
Medication Adherence Analytics
Meet the most powerful single-vendor Medication Adherence and Smart Package Lifecycle System in the World:
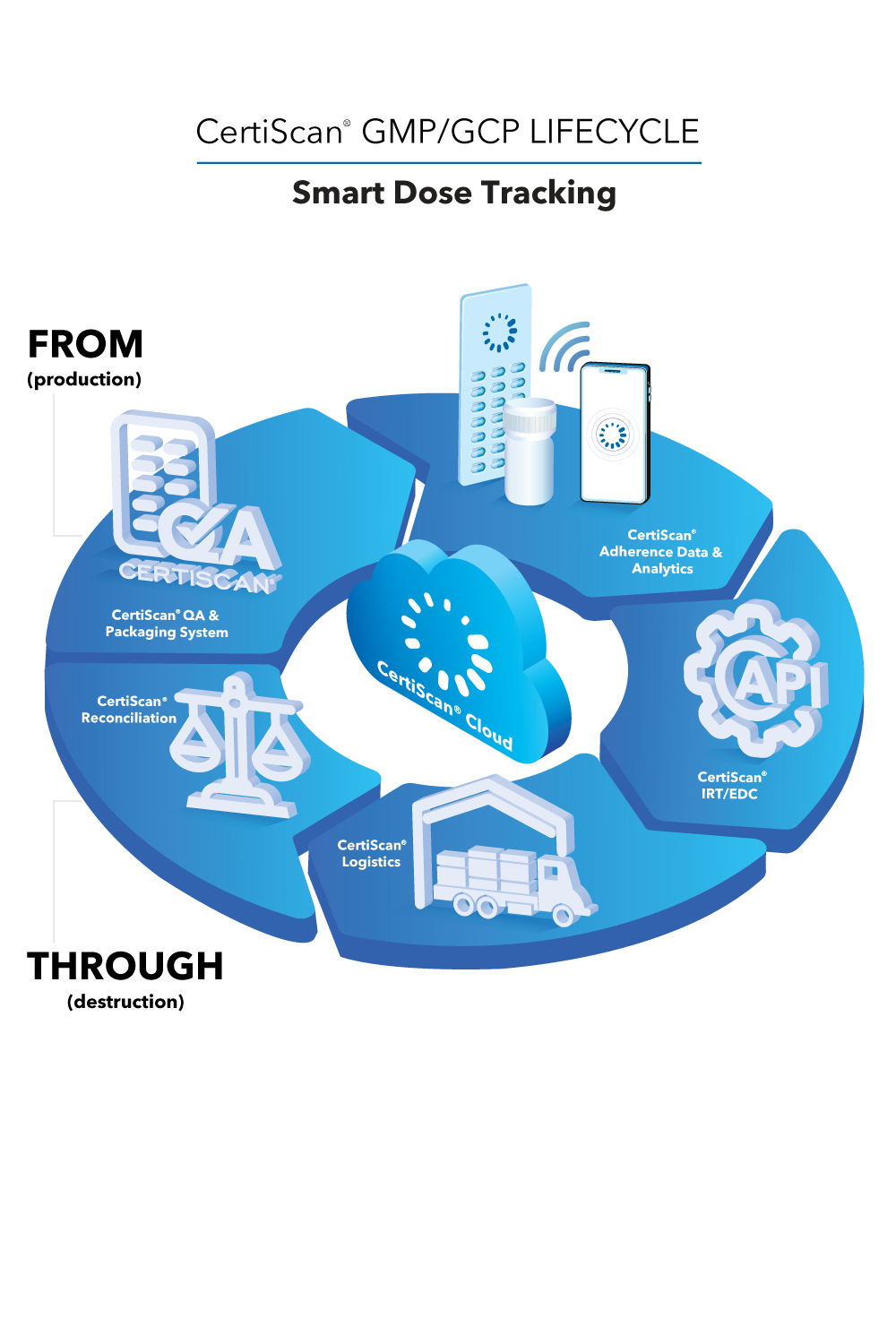
CertiScan® GMP/GCP Cloud: Dose-Level Lifecycle Data, Analytics, IRT, Reconciliation; all connected to the best-in-class adherence devices from Information Mediary Corp. Our adherence devices place the power to avoid narrowly missed endpoints in your patients’ hands.
CertiScan® Cloud is an innovative SaaS where GMP (devices) meets GCP (data and analytics). CertiScan® Cloud provides dose-level traceability of drug supply from packaging to dispensation and destruction – combining it with patient dose taking adherence insights, making it a proven, strong and insightful tool for successful drug research.
CertiScan® Cloud easily integrates with partner IRT and EDC systems, and is compliant with HIPAA, GDPR, 21 CFR Part 11, and EU Annex 11.
Med-ic® is the only Smart Dose blister ever submitted with a FDA priority review resulting in a blockbuster drug approval.
Other award-winning devices fully integrated in this amazing ecosystem:
- Med-ic® Smart Blister and Vial Package
- eCAPTM Smart Pill Bottles
- CoolBlue®-AI Smart Syringe Unit Package
- eBOXTM Smart MultiMed Box
- Med-ic® Polypharmacy Card
- CoolBlue®-AI Intelligent Temperature Monitoring
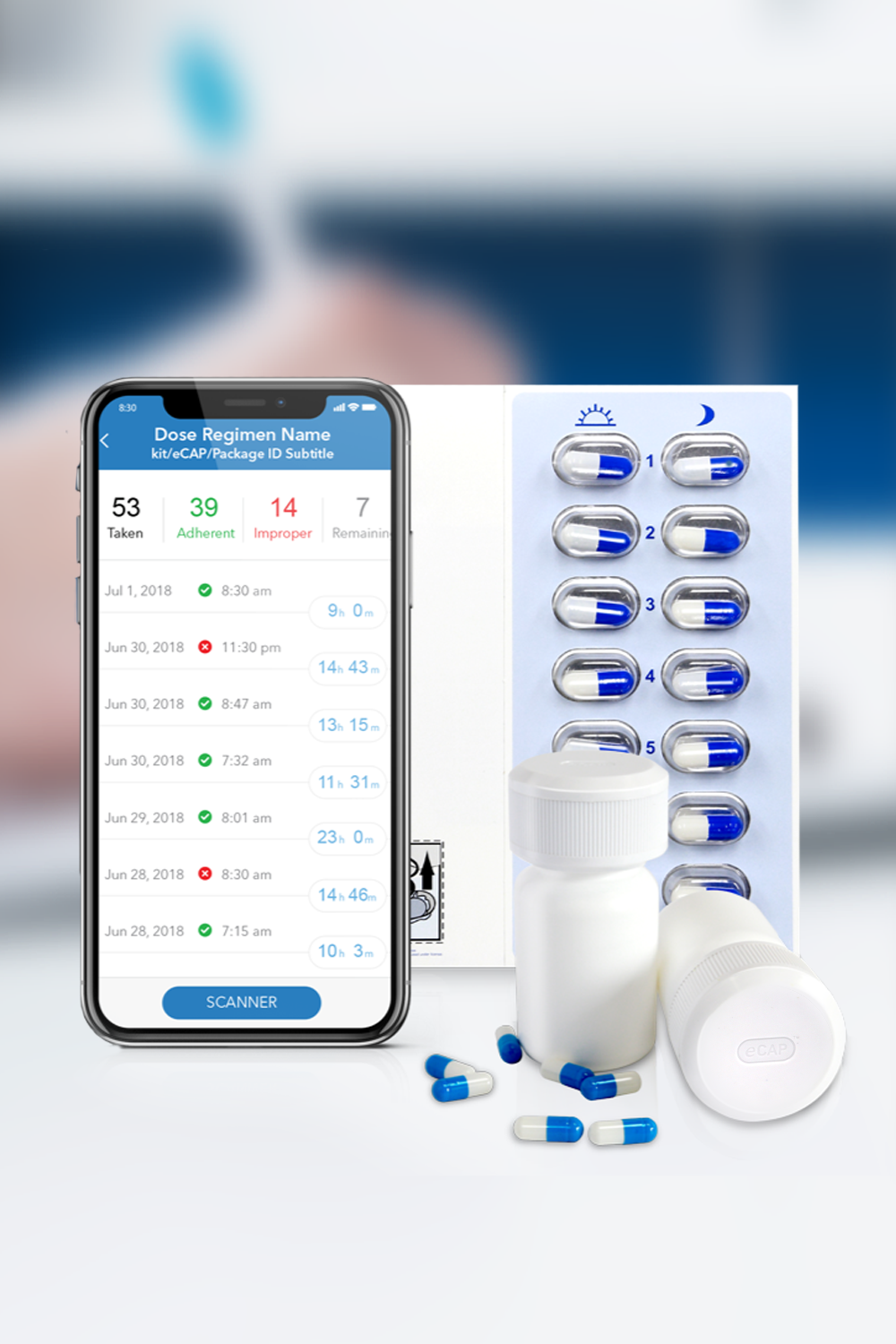
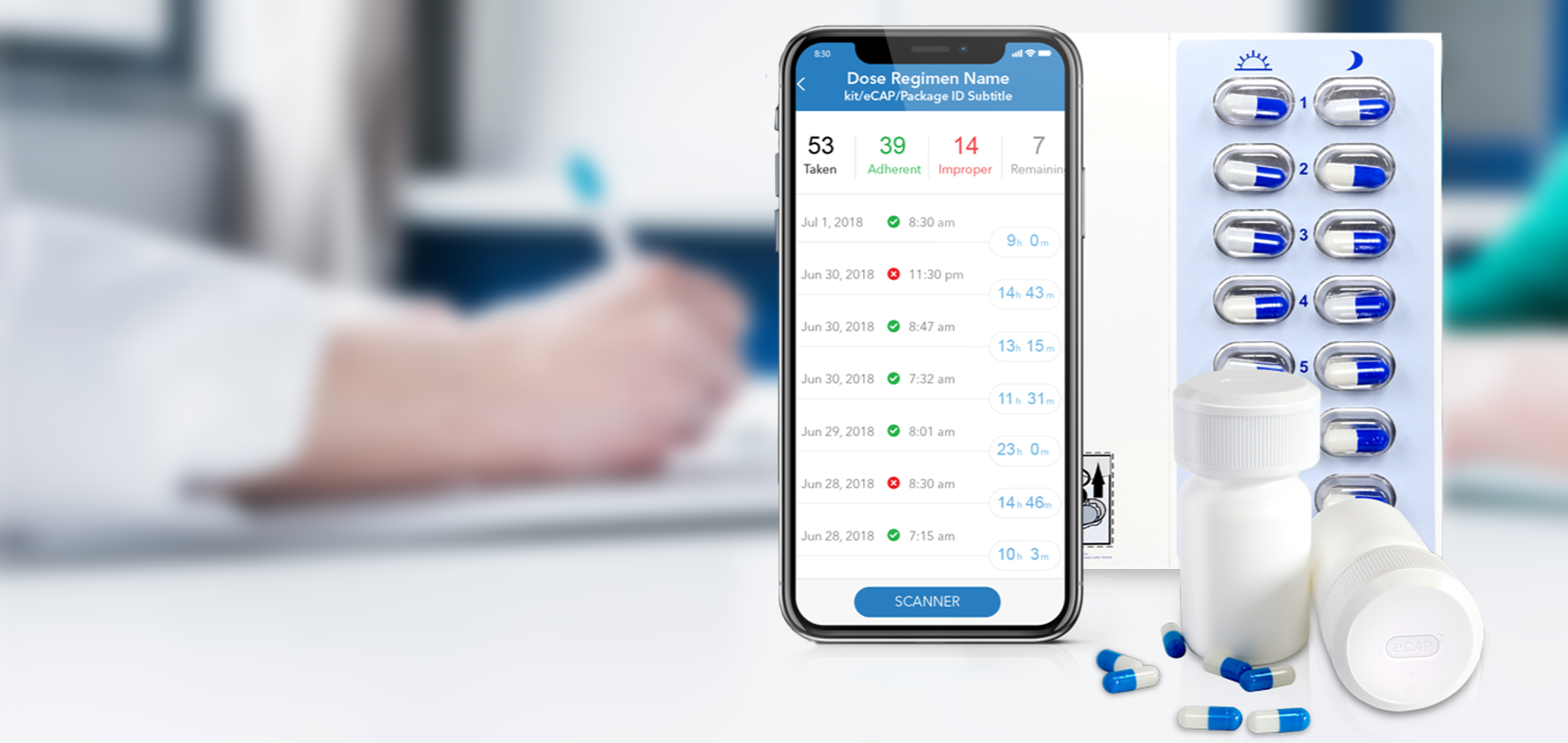
Medication Adherence Matters
Return on Investment in Research
Clinical decisions (both human and AI) should be based on 100% adherence with the medication regimen, something rarely achieved in outpatient settings.
Our ECMs improve research efficiency and accuracy by mitigating non-adherence and leading to improved outcomes.

Medication Adherence Matters
Benefits in Digital Health
Insight into your patient’s medication adherence is invaluable. Discover how our industry leading Digital Health solutions lead to improved medication adherence, better clinical outcomes, fewer patient dropouts and even reduced clinical trial sizes.
Our Med-ic® smart blister package and eCAPTM smart pill bottle provide medication adherence data – an indispensable part of any clinical decision.

Latest News
IMC sets new clinical research standard with CertiScan® 2.0 end-to-end medication adherence analytics, disrupting smart device marketplace with customer-friendly ROI-driven data plans
29.04.2021
Att: Health Tech Desk FOR IMMEDIATE RELEASE April 28, 2021 New York, NY Contact: Joanne Watters – jwatters@informationmediary.com IMC sets new clinical research standard with CertiScan® 2.0 end-to-end medication adherence analytics, disrupting […]
Events | Apr 26-29, 2021
The GCSG 2021 US Virtual Conference
24.03.2021
Knowledge Papers
DRIVING ADHERENCE WITH SMART MEDICATION PACKAGING SOLUTIONS
02.12.2019
Imagine if a package could know the time each dose was taken and use this information to drive customized reminders, in-app coaching and data analytics! That’s the power of Med-ic®, […]
Adherence Insights
Our Most Frequently Asked Question
04.03.2019
The most frequently asked question we get is “Can you do real-time adherence monitoring?” The simple answer is “yes, but… how will you use real time data in real time?” […]
Knowledge Papers
Making trials smarter
18.03.2019
Electronic content monitors (ECM) add cost to a clinical trial but offer enormous return on the investment (ROI). This is why progressive clinical trials are using our CertiScan ECM toolKIT […]
Latest News
IMC Partners with Medopad
20.03.2019
Information Mediary Corp Joins Medopad Global Digital Health Platform London, UK and Ottawa, Canada – 22 October 2018 – Medopad, a health technology company with partners such as Apple (NASDAQ: […]
Latest News
World’s First and Only NFC Forum Certified Adherence Solution
24.07.2019
IMC, the global leader in Smart Medication Adherence Solutions, is proud to announce that our Electronic Content Monitoring NFC Tag is the world’s only Medication Adherence Device certified and listed […]