Evaluating Hepatitis C Treatment in Phase 4 Trials
When an international team needed a precise way to measure adherence, they turned to Med-ic smart blister packages. Their findings have practical clinical applications, and also demonstrate the advantages of smart packages versus clinical pill counts or participant self-reports.
Overview
Direct-Acting Antiviral (DAA) therapy is an effective treatment for hepatitis C. However DAA is expensive, and patients who use drugs or alcohol face barriers to accessing DAA. With the support of AbbVie and Gilead, an international team sought to understand the patterns of drug and alcohol usage and their impacts on DAA usage, including factors associated with nonadherence and the impact of adherence on Sustained Virologic Response (SVR). They carried out 2 international, multicenter, open-label, single-arm, phase 4 trials: D3FEAT and SIMPLIFY.
Challenges
The majority of studies evaluating adherence among people with recent injection drug use or receiving opioid agonist treatment had used imprecise methods for measuring adherence.
Solution
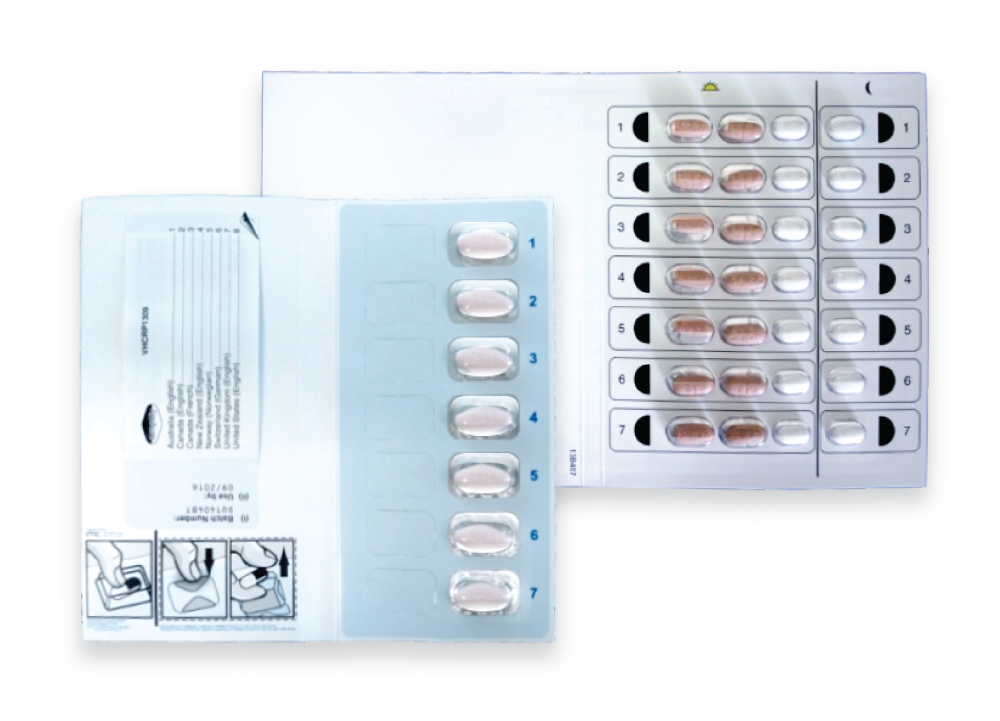
Med-ic smart blister packages were used to track dose dates and times. These Med-ic packages were custom designed and fit-for-purpose: they contained 1 tablet per day in a single blister in SIMPLIFY, and 3 tablets in individual blisters for the morning dose and 1 tablet in a single blister for the evening dose in D3FEAT. Participants received AUS$10 (or equivalent) to return each blister pack. Adherence was also measured by counting remaining pills in the returned blister packs (clinical pill count) and through self-reported adherence questionnaires every 4 weeks. Participants completed a self-administered questionnaire on a tablet computer at enrollment, at treatment commencement, and every fourth week during treatment.
Results
The use of Med-ic smart blister packs for adherence monitoring was a major strength of this study, allowing for detailed and accurate adherence measurement over time, providing a more precise estimate of the effect of time on nonadherence.
Using Med-ic and IMC solutions, the researchers were able to reach the following conclusions:
- This study demonstrated high adherence to once and twice-daily DAA therapy among people with recent with recent injection drug use or receiving opioid agonist treatment.
- The observed nonadherence did not impact treatment or outcomes, suggesting forgiveness to nonadherence.
- These data are important to inform clinical guidelines, clinical management, and health policy, particularly in settings where there are restrictions for the reimbursement of DAA therapy for people who inject drugs.
- Blister pack measurement of adherence remains a more robust method of measuring adherence compared to clinical pill count or self-report
Interested in learning more about this case? <a href="/contact-us">Contact us</a>.