
PRESS RELEASE May 6, 2024 – New York, NY — Information Mediary Corp (IMC)…
Read More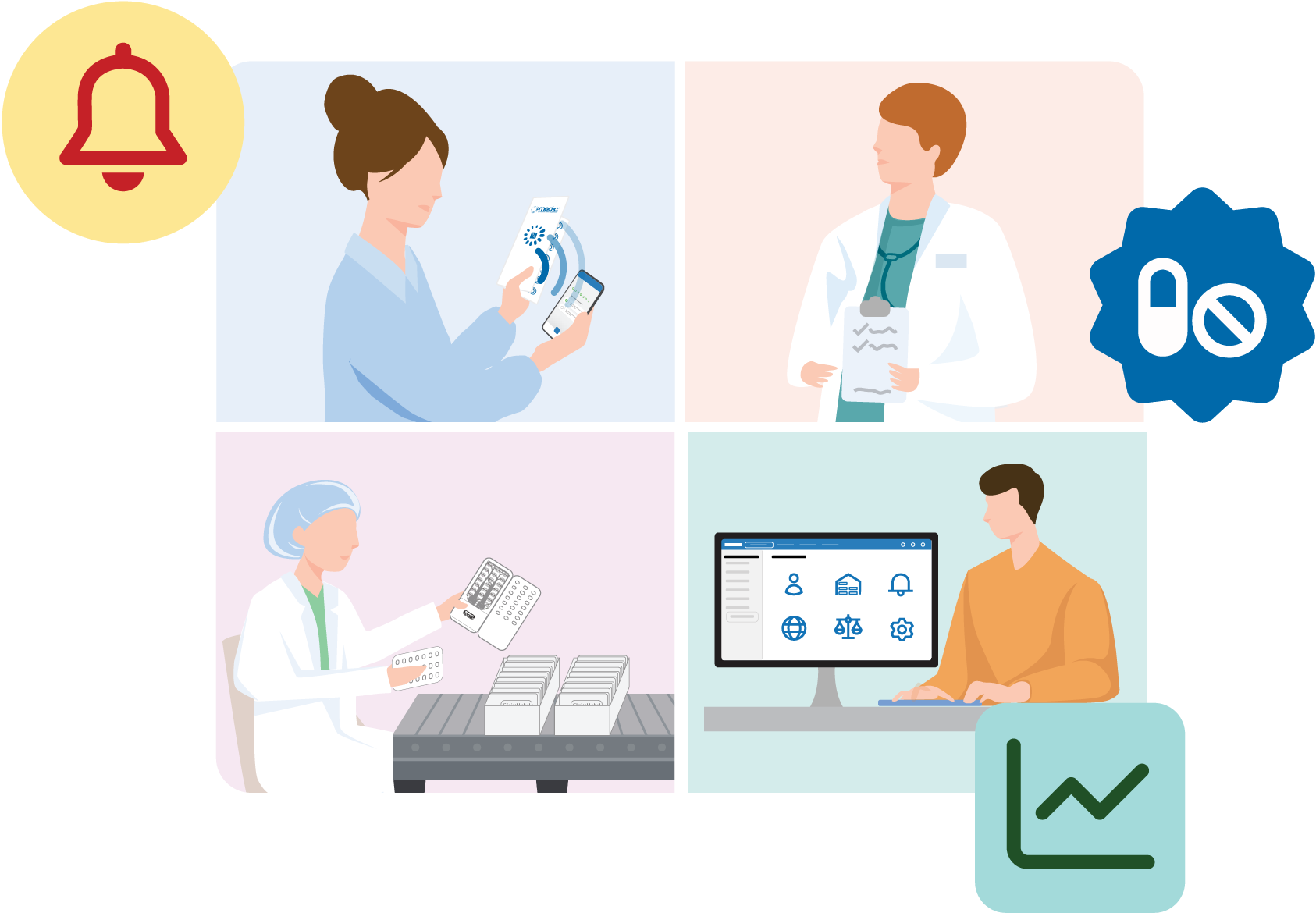
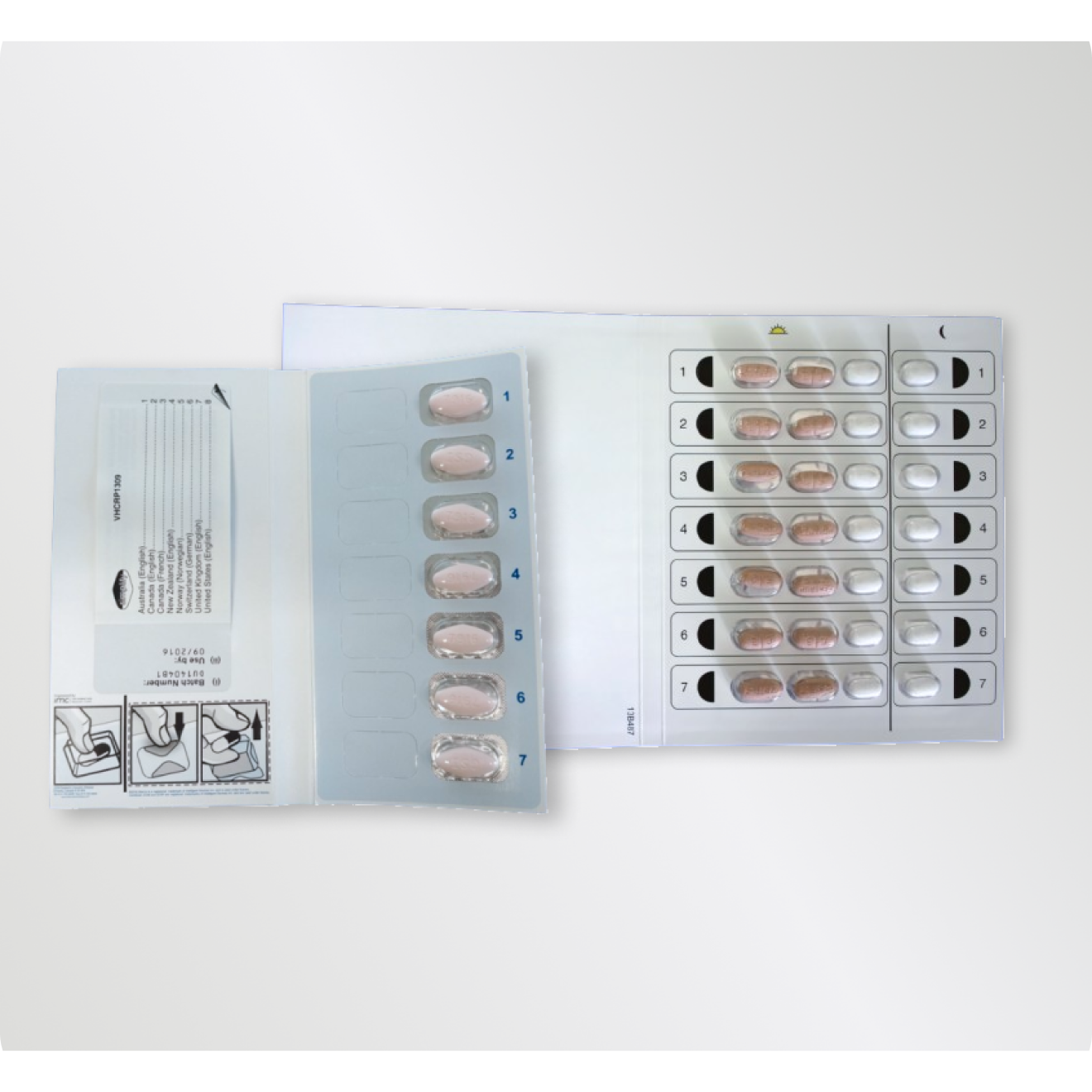
Overview Direct-Acting Antiviral (DAA) therapy is an effective treatment for hepatitis C. However DAA…
Read More

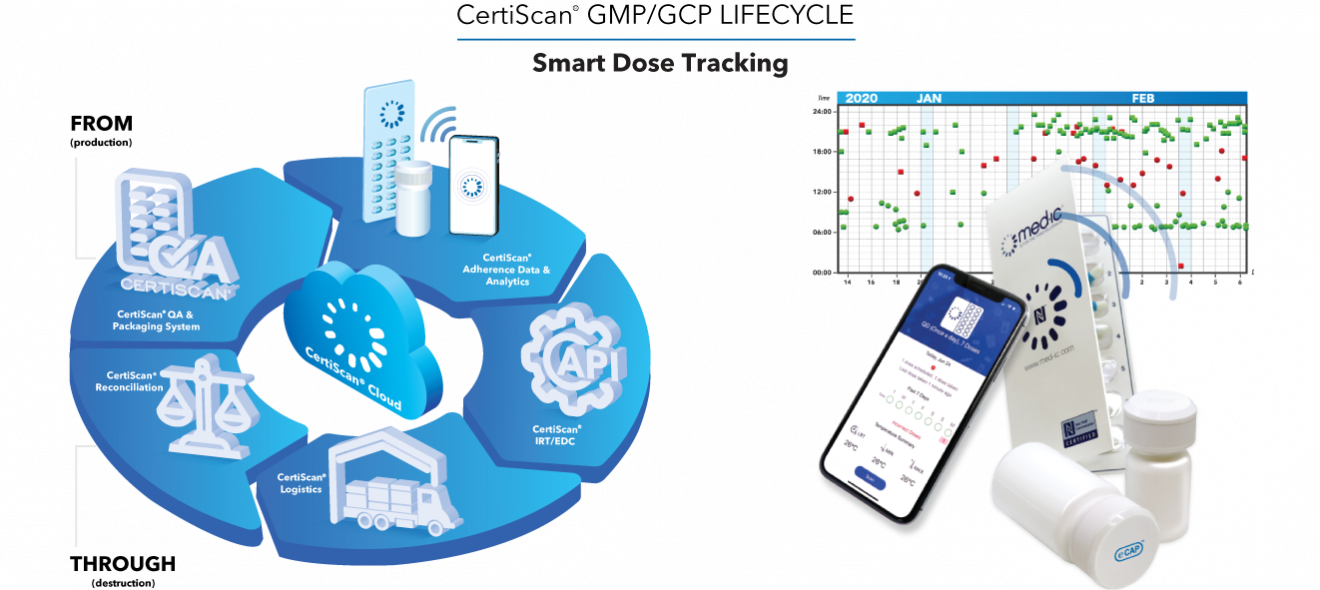
Att: Health Tech Desk FOR IMMEDIATE RELEASE April 28, 2021 New York, NY Contact:…
Read More