Sponsors

Solutions
Learn More about the Products
that Power our Solutions


ROI
Direct Savings from Reduced N

Additional Weeks of Patent Protection

Compensate for Low or Slow Enrollments
Accurate data for transformative solutions.
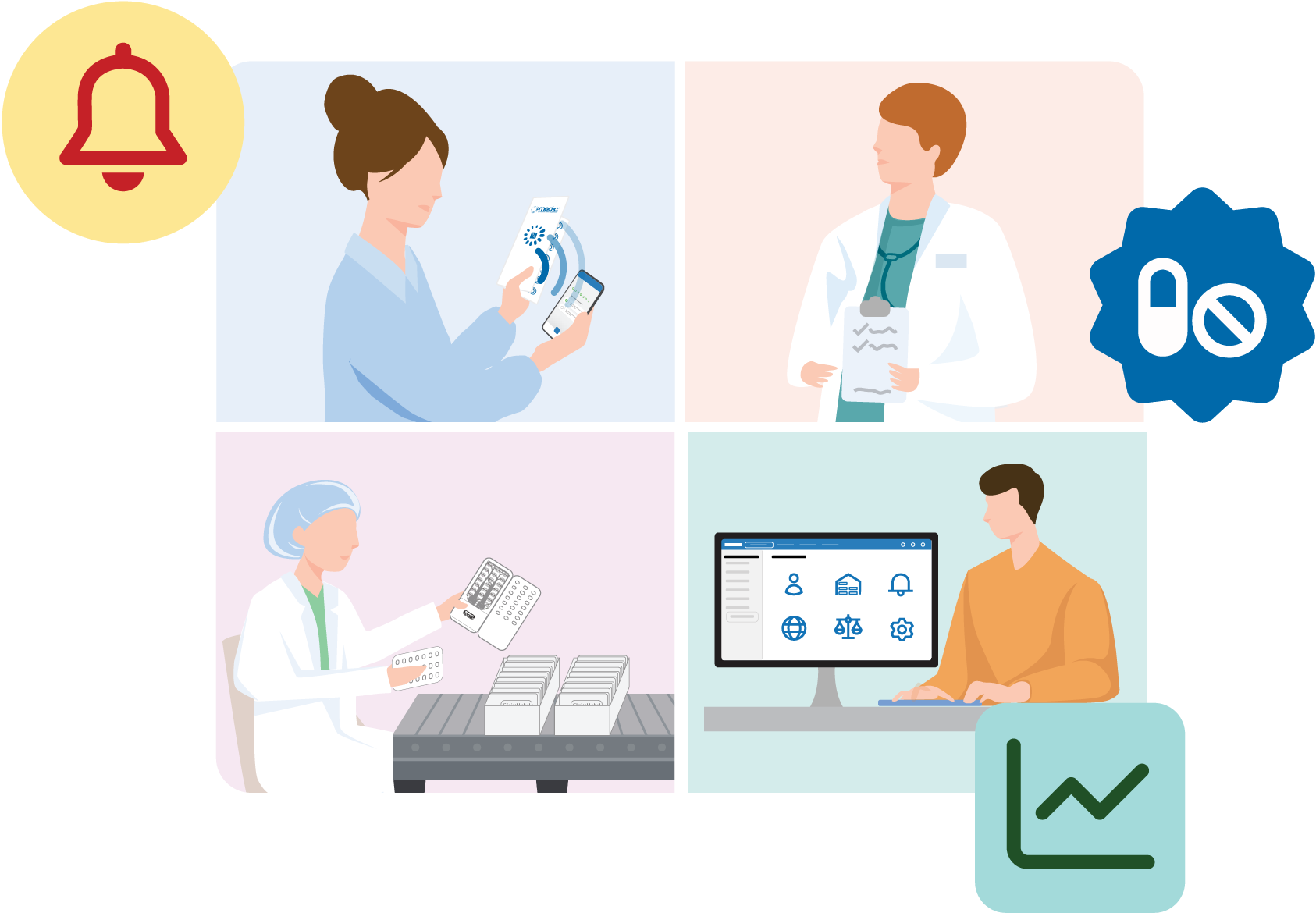
Inappropriate medication use is widespread in clinical research. This can harm individual participant outcomes, jeopardize trial outcomes, and cause hidden errors that are only discovered post market or after publishing (such as a poorly titrated dosage or undiscovered side effect).
Our solutions make it easy to detect and treat these harmful issues. Many protective features are automatic, such as mobile reminders, misuse alerts, targeted coaching.- Medication non-adherence
- Medication misuse or abuse
- Temperature exposure and spoilage
- Expiry
- Tampering, fraud, and other lot or batch issues
Shorter trials that reach their endpoints more quickly can gain weeks or months of additional patent protection. This can amount to substantial additional revenue.
Our solutions help studies reach last dose, last participant sooner.- Automating data collection
- Replacing diaries and ePRO with smart packaging
- Letting CertiScan preprocess and analyze data
- Integrating CertiScan with RTSMs, EDCs, and other platforms
- Conducting adaptive trials
Clinical trials are becoming increasingly complex and expensive. Certain activities in a protocol seem straightforward on paper, but in practice can translate into dozens of hours of site work.
Use our solutions to help eliminate needless overhead and streamline processes.- Automating data collection
- Basing site inventories on consumption rates
- Conducting adaptive trials
- Conducting decentralized trials
New, technology-first strategies are transforming clinical trials. DCTs, participant diversity initiatives, and AI tools are changing from “nice-to-haves” into “must-haves”.
Our solutions are modern tools for modern trials that can help researchers and other stakeholders gain and maintain an advantage.- Meet FDA trial modernization guidelines
- Model PK and PD with adherence data
- Satisfy internal and external innovation requirements
- Build accurate, objective datasets for use in AI models, participant diversity initiatives, DCT tools, and more
- Publish unique studies that stand out in the literature