Putting The “Scan” in CertiScan: How To Download Data from Smart Packages
Learn more about our integration options and developer platform.
 19 July 2024
19 July 2024
We support several data retrieval schemes, each tailored to a specific use case. Read on to learn why you may prefer one versus another, and the benefits for your program.
Smart blister packages and bottle caps are the ideal adherence data source. They are accurate, objective, automated, and passive. There is no extra effort on the participant’s part since they were going to take a dose anyways (or not - which smart packaging can also detect!).
“No extra effort” is a vital point that’s worth pondering for a moment. As soon as a participant removes a dose from their Med-ic® smart blister package or eCap™ smart bottle cap, the information is permanently stored on the electronics. It can be downloaded immediately or at a later time.
In the words of Mark Butler, eCap™ user and Clinical Psychologist at Northwell Health: “It consistently measures data regardless of input from the participant so you’re reducing burden on them”.
How is this dosing data downloaded? It’s a simple matter of performing a “scan”: bring the smart package close to a mobile phone or desktop reader device. Scanning is based on near-field communication (NFC), the secure wireless technology that powers tap to pay services (such as Apple Pay and Google Pay).
After this effortless scan, the dosing data feeds into the CertiScan® Adherence Platform and other software systems to power numerous solutions.
We support different data retrieval schemes, each specific to a certain use case. Your use case depends on your response to the question: “How will I use the dosing data, and what value do I hope to extract from it?” In general there are 4 use cases…
Use Case 1
“I want to analyze dosing data in real-time and use it to assist participants with remote interventions”

Your participants should use the CertiScan® mobile app. Participants take their doses and scan their smart packaging. Download and analysis are instantaneous on the device: they can see a daily schedule of medications they are supposed to take according to their protocol, and this will automatically be filled out by the smart package data, no manual interactions or screen inputs necessary! The CertiScan® mobile app will synchronize all of this information and analysis with a central server for various clinical trial stakeholders to monitor and engage with remotely.
Use Case 2
“I want to analyze dosing data in real-time but my protocol will not involve remote participant interventions”

Your participants should use the CertiScan® mobile app in a blinded mode. There will be no daily schedule, reminders, coaching alerts, or other bells and whistles. The user interface will be minimized down to a simple instruction to “scan”, a button, and a straightforward status message to confirm that the scan succeeded and the dosing data was received by the central server.
Use Case 3
“I want to analyze dosing data at regular intervals during each participant’s enrollment period, but real-time isn’t necessary”
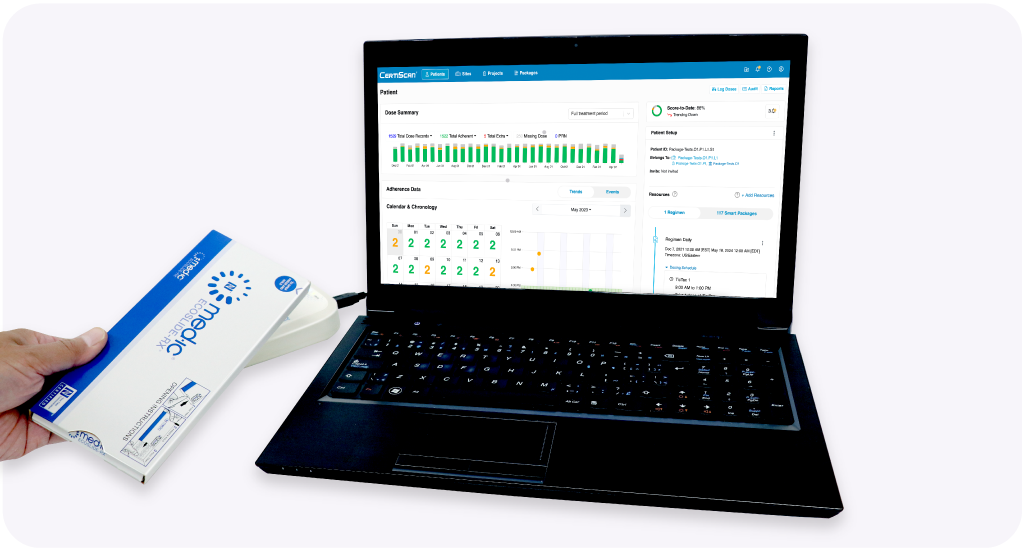
Whenever participants return to the site, your research team should use CertiScan® Connect to wirelessly download the package data. Data is automatically analyzed and visualized in the CertiScan® study portal for the site staff members to review during the participant visit and afterwards. It’s possible to review the data with the participant during the appointment, showing the easy to understand graphics and visualizations, pointing out good adherence to provide reassurance and reinforcement, or areas of improvement.
Use Case 4
“I will only analyze the participant’s dosing data after they have finished their course of treatment; there is no need to analyze at intervals”

Participants return their used smart packaging to a predetermined location to be scanned. Depending on your study, this involves an in-person return visit to the site site, or it could involve mailing the used packaging to a central scanning depot where operators perform the scan and download the dosing data. This may be the least burdensome and simplest method for some studies, visualizations, pointing out good adherence to provide reassurance and reinforcement, or areas of improvement.
Bonus Use Case
“Can I do a combination? Real time for some trial arms or some participants and post hoc for others?”
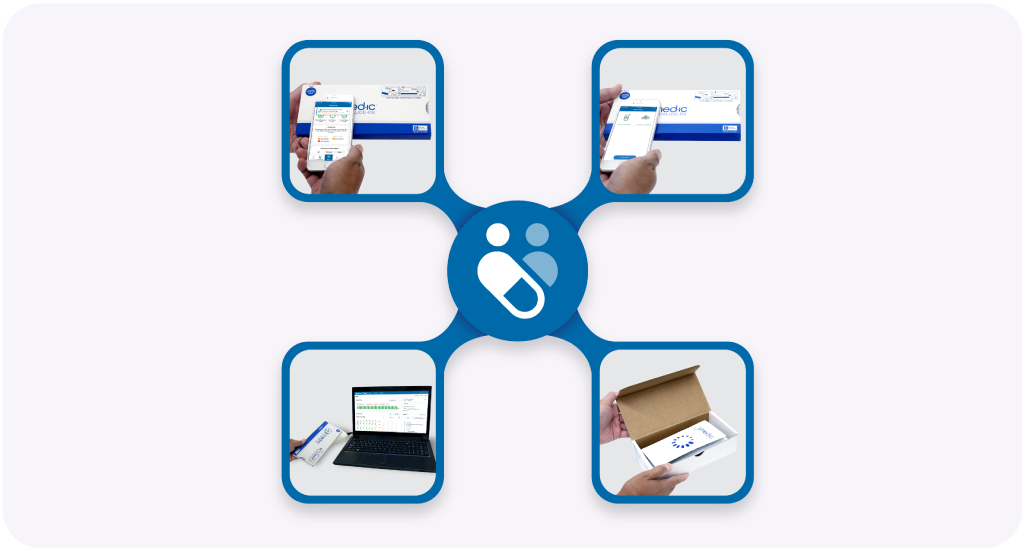
Absolutely! All scanning methods can be mixed and matched to create a data acquisition solution that will be fit for your purposes.
Our Take
The decision on any of the above scanning schemes will depend on your needs and your study protocol. We strongly believe that the highest value of our technology comes from using adherence data as soon as possible to help counsel and guide each participant towards optimal adherence, since optimal adherence leads to optimal outcomes (both for the individual participant and for the overall trial).
Regardless of your decision, we have robust and feature complete technology to assist you in reaching your objectives.There is a tremendous ROI to be realized.
Ready to start? Contact our team to begin the conversation.
Getting Advanced and Going Further
Does your trial already use mobile apps? Do you want to integrate a clinical trial management system with smart package data? We offer the CertiScan® SDK - a software package that lets you download and process smart packaging data within existing apps - and CertiScan® Cloud APIs - that let you integrate our data with your RTSMs and EDCs.
Learn more about our integration options and developer platform.